Yeah, you read the title correctly and no, i haven't gone crazy. There are actually 6 states of matter and not only the traditional 3 states of matter that you may have read when you were in school.
So, now you may be wondering what are the other 4 states of matter and well I'm here to tell you just that.
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. The volume is definite if the temperature and pressure are constant. When a solid is heated above its melting point, it becomes liquid, given that the pressure is higher than the triple point of the substance.
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough kinetic energy so that the effect of inter-molecular forces is small (or zero for an ideal gas), and the typical distance between neighboring molecules is much greater than the molecular size. A gas has no definite shape or volume, but occupies the entire container in which it is confined.
So, now you may be wondering what are the other 4 states of matter and well I'm here to tell you just that.
Various States of Matter
So, now you know that there is more than one state of matter. But have you ever wondered what makes each state of matter different from each other...
Well if you have read chemistry in your high school and college you might know that the state of matter depends on the speed of the movement of the particle's and the space between the particles...For Example, in solids the particles are closely packed together and the vibrate about in their own positions. Whereas in gases there is a lot of space between the particles and the particles can freely move around.
Now lets know a little more about the various other states of matter...
1. Solid
In a solid the particles (ions, atoms or molecules) are closely packed together. The forces between particles
are strong so that the particles cannot move freely but can only
vibrate. As a result, a solid has a stable, definite shape, and a
definite volume. Solids can only change their shape by force, as when
broken or cut.
2. Liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. The volume is definite if the temperature and pressure are constant. When a solid is heated above its melting point, it becomes liquid, given that the pressure is higher than the triple point of the substance.
3. Gas
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough kinetic energy so that the effect of inter-molecular forces is small (or zero for an ideal gas), and the typical distance between neighboring molecules is much greater than the molecular size. A gas has no definite shape or volume, but occupies the entire container in which it is confined.
4. Plasma
Plasma is the fourth state of matter.
To put it very simply, a plasma is an ionized gas, a gas into which
sufficient energy is provided to free electrons
from atoms or molecules and to allow both species, ions and electrons,
to coexist. The funny thing about that is, that as far as we know,
plasma is the most common state of matter in the universe. They are
even common here on earth. A plasma is a gas that has been energized to
the point that some of the electrons break free from, but travel with,
their nucleus. Gases can become plasma in several ways, but all include
pumping the gas with energy. A spark in a gas will create a plasma.
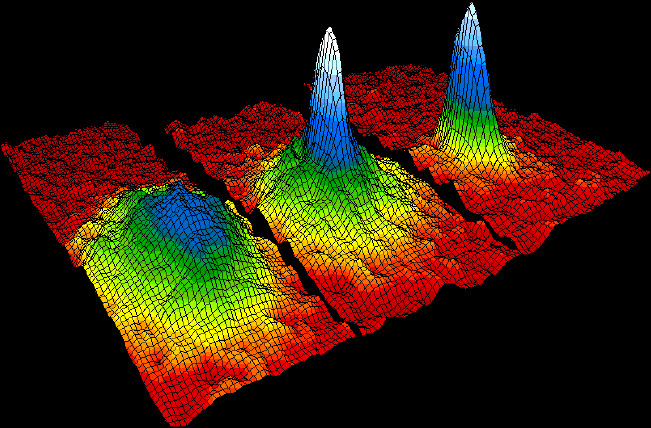
5. Bose Einstein Condensate
A Bose–Einstein condensate (BEC) is a state of matter of a dilute gas of bosons cooled to temperatures very close to absolute zero (that is, very near 0 K or −273.15 °C). Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which point macroscopic quantum phenomena
become apparent. It is formed by cooling a gas of extremely low
density, about one-hundred-thousandth the density of normal air, to
ultra-low temperatures.
6. Quark - Guluon Plasma
A quark–gluon plasma (QGP) or quark soup is a state of matter in quantum chromodynamics
(QCD) which is hypothesized to exist at extremely high temperature,
density, or both temperature and density. This state is thought to
consist of asymptotically free quarks and gluons,
which are several of the basic building blocks of matter. It is
believed that up to a few milliseconds after the Big Bang, known as the Quark epoch,
the Universe was in a quark–gluon plasma state. In June 2015, an
international team of physicists produced quark-gluon plasma at the Large Hadron Collider
by colliding protons with lead nuclei at high energy inside the
supercollider’s Compact Muon Solenoid detector. They also discovered
that this new state of matter behaves like a fluid.